How do we classify matter? One of the ways, one that most of us are familiar with, is in terms of “the state of matter.” Is it a solid, a liquid, or a gas? If you take some ice cubes, a solid, and stick them inside a bottle they maintain their ice cube form. They are unperturbed by the fact that they are in the bottle. If you melt the ice cubes, they become water, a liquid, and take on the shape of the bottle, but remain confined by gravity to the bottom of the bottle. If you vaporize the water into steam, a gas, the steam fills the entire bottle and conforms to its shape completely. Actually there is a fourth state of matter to consider. If you ionize the water by ripping off its valence electrons and maybe even some of the lower energy ones, it become a charged gas, referred to as a plasma.
At an atomic level, the atoms in a gas are largely oblivious of one another. This is because they seldom come in contact with one another. Their interactions, with say light, are single atom events and independent of what the other atoms are doing. In a liquid the atoms start to interact with one another. In a solid the interaction is complete. The atoms form well defined structures called crystals. The crystal now interacts with light as a unit. As a result, we talk about the energy levels of the crystal, no longer of the individual atoms.
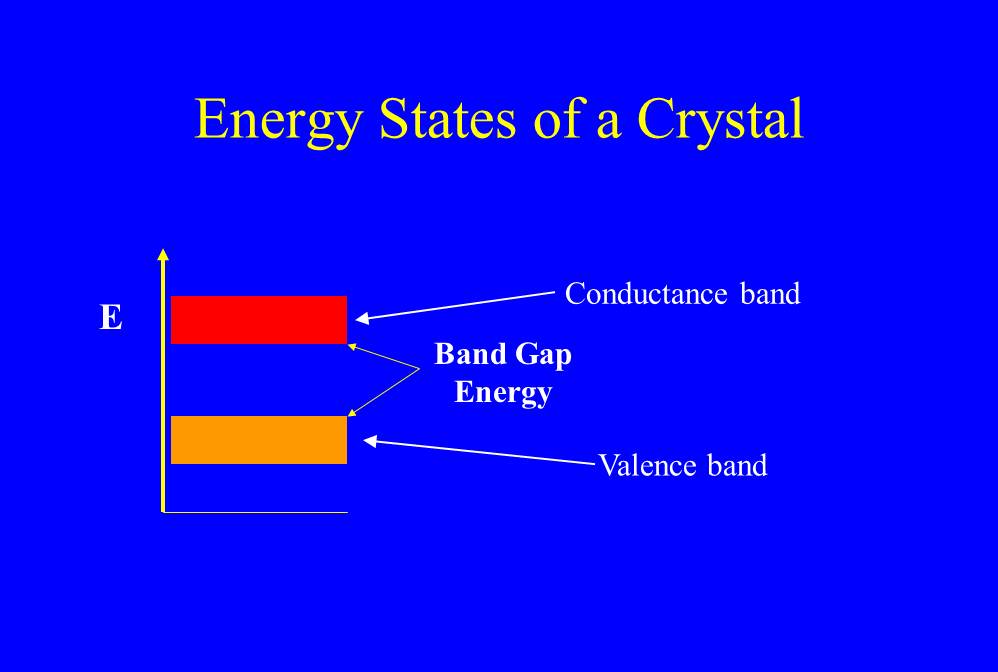
Because of this cooperative effect, the energy states start to blur out and become a continuum. However, there is still an energy gap between the states of bound valence electrons, called the valence band [of energy levels], and the energies of liberated or free electrons, called the [conductance band]. I’ve illustrated this schematically in Figure 1.
(BTW Figure 1 gives you the opportunity to thumb your nose at your high school or physics college teacher, who always insisted that you label axes of a graph and marked you down if you didn’t. The y axis is energy, but there is no x axis label. This diagram is called a Jablonski diagram in physics.)
How does an electron gain the energy to escape from the valence band to the conductance band in a crystal. It can be done with heat, or electrical energy, or with light. A photon of light can be absorbed and give the electron enough energy to escape.
You can define three types of solid materials on the basis of how easy it is to liberate the valence electrons. In conductors, typically metals, the band gap energy is so small that it occurs very easily at room temperature. Many electrons go into the conductance band, and if you attach the conductor across the terminals of a battery a current readily develops. In insulators, the band gap is huge and precious few electrons are in the conduction band. The material does not conduct electrons. Finally, there are in between materials, called semiconductors, which need a boast to get the valence electrons into conduction band.
One such boast, as we’ve already described, is light. This kind of semiconductor/ light interaction is the basis of both analogue and digital photography. As we will see, it explains how silver halide films work, how camera light meters work, and how digital camera sensors work.
In the next few technical blogs, I’d like to explore the inner workings of the silver halide film process. Breaking it all down, we need to sequentially consider:
- What is a silver halide?
- What is a photographic emulsion?
- How is the latent image formed?
- How is the latent image developed?
- How is the developed image fixed or prevented from further interaction with light?