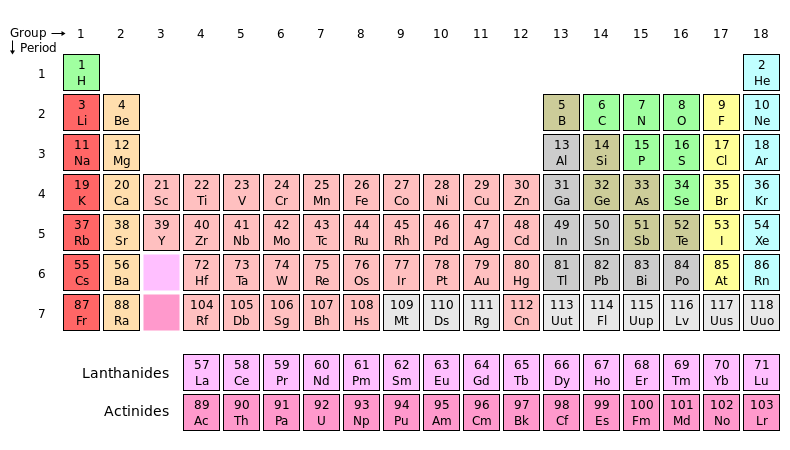
Figure 1 – The Periodic Table of the Elements. The halide group is shown in yellow. From the Wikicommons and in the public domain.
In considering silver halide chemistry, the first question is exactly what is a silver halide. A silver halide is a compound formed from silver and a halogen. That didn’t get us very far. What’s a halogen? If we look at the period table of the elements (be sure to click on Tom Lehrer’s version) in Figure 1, the yellow group 17 are the halogen elements: Fluorine, Chlorine, Bromine, Iodine, and Antimony.
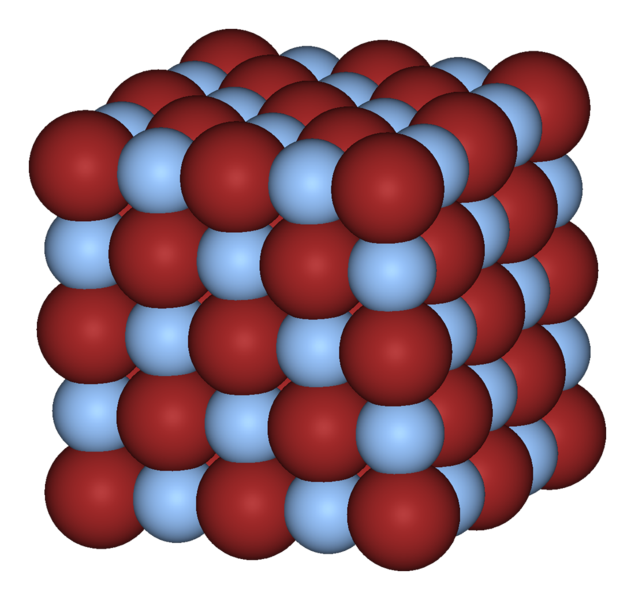
Figure 2 – The crystal structure of silver bromide. The silver atoms are the red spheres the bromide atoms are the pale blue spheres. Form the Wikicommons and in the public domain.
A silver halide then is a crystal formed between silver and one of these Group 17 elements. Without getting into too much detail the silver metal gives away some of its electrons to become positively charged. These are scarfed up by the halogen to become negatively charged. The halogens ions all have a charge of -1 (electron’s worth of charge). That’s why they are grouped together. Since opposites attract the silver and halogen ions attract one another and form very compact and structurally well-defined crystal structures. These crystals typically take on a cubic form as shown in Figure 2, for the important photographic material silver bromide, where the red spheres are the silver atoms and the pale blue ones are the bromine atoms. The other common photographic silver halides are silver chloride and silver iodide.
Silver bromide is not soluble in water. This is convenient. If you mix a solution of silver nitrate in water with potassium bromide in water, the silver and bromine switch partners to form silver bromide and potassium nitrate. The potassium nitrate stays in solution. The silver bromide precipitates into fine silver bromide crystals, ready to be washed and put into a photographic emulsion. What an emulsion is will be the subject of my next technical blog.
