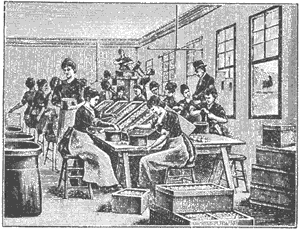
Figure 1 – Women separating egg whites and egg yolks as the first step in the manufacture of silver albumen photographic paper from Josef Maria Eder’s Ausführliches Handbuch der Photographie, Book IV, part 1, 1898 edition. In the public domain in the US. See hyperlink in text for more information on the process.
A photographic emulsion is a fine suspension of insoluble light-sensitive crystals, for example silver bromide, suspended in a colloidal solution. Basically, this means that while the crystals are not themselves soluble in water, they can be suspended in a material, such as gelatin, which is then allowed to harden thereby suspending the crystals permanently.
The emulsion is layered onto a substrate such as glass or a film, e.g. nitrocellulose or plastic. In the nineteenth century the emulsion material was most often egg albumen, and this created an entire industry of manufacture that started with the separation of thousands of egg yolks and whites as shown in Figure 1. Modern photographic emulsions are made from gelatin, which is partially hydrolyzed (boiled) extract of animal cartilage and bone – hence the classification of silver-gelatin print.
The light sensitive emulsion is generally manufactured, as suggested in my previous blog by dissolving silver nitrate and potassium bromide in a hot gelatin solution and allowing the crystals of silver bromide to precipitate out in the gelatin.
So now, we have the fundamental elements of a photosensitive material. You can do this yourself at home, if you are industrious, and there are even commercial kits to help you. If you want to do albumen printing you can purchase kits from Bostick and Sullivan. As should be obvious from our discussion of color photography and color movies, the fundamental silver halide process is ubiquitous.
Curiously, how it works, what the fundamental mechanism of light sensitivity is, was largely a mystery for the first century of photography. That mystery is the subject of my next technical blog.
