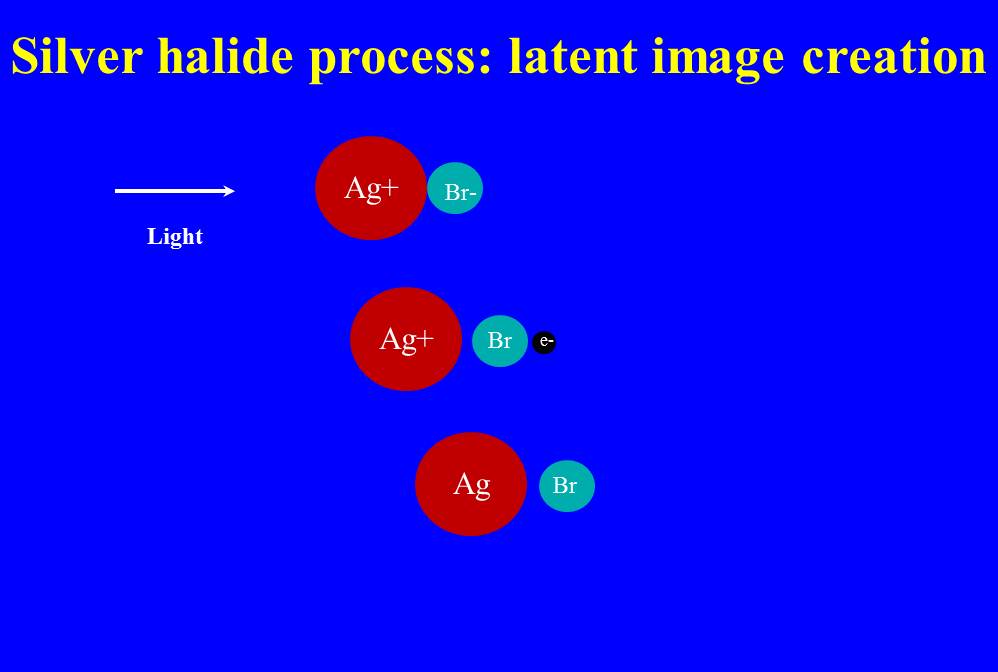
FIgure 1 – Schematic showing the creation of a latent image upon exposure to light in a silver bromide emulsion. (c) 2013 DE Wolf
You will recall that photography was invented in 1838. So the light sensitivity of silver salts, such as silver bromide, was recognized for a very long time.* However, it was not until 1938 that a real theoretical-mechanistic explanation of this sensitivity began to be developed.+ We have already discussed the concept of valence and conduction bands in silver halides and how light can raise electrons between these two bands. Recognize however, that when the electron is elevated in energy to the conduction band it leaves behind a positively charged region in the crystal lattice, referred to as a “hole.” In fact, what light actually creates is an electron-hole pair.
In a perfect and pure crystal the electron will hang out a bit in the conduction band and eventually will recombine with one of the available holes. So there should be this continuous up-down, creation-recombination process going on in the presence of light. However, our photographic grains are not perfect crystals. First, of all they are finite in size. Second, they have impurities. And third, there can be breaks or physical dislocations in the crystal lattice – like cracks in a driveway.
All of these imperfections can lead to the process shown in Figure 1. Here, I’ve shown it as a single bond between a silver ion and a bromine ion, but we know that in fact the ionic interactions are three dimensional and more complex. We have, for starters, a silver ion (Ag+) in close association with a bromine ion (Br-). Light comes in and liberates the bromine’s electron. So now we have a silver ion (Ag+) and bromine atom (Br) and an electron (e-). The electron then combines with the silver ion to create atomic silver (Ag). So we are left with silver (Ag) and bromine (Br). It is the free atomic silver that we refer to as the latent image.
In the emulsion there are thousands of randomly distributed silver bromide crystals or grains. These are the pixels of analogue photography. Where light struck the emulsion some of these grains now contain free silver. The more intense the light the more likely the grain is to contain free silver. The distribution of silver reflects the distribution of light and is what we refer to as “the latent image.” You can’t see it and if you turn the lights on to try to see it, you will over expose the film – “Catch 22!”
The latent image is there waiting to be developed. That will be the subject of my next technical blog.
+Gurney, R. W.; Mott, N. F. (1938). “The theory of the photolysis of silver bromide and the photographic latent image”. Proc. Roy. Soc. A164: 151–167.
