Today, I’d like to begin a discussion of the physics behind light detection. By light detection I mean both how analogue films work and how digital camera sensors work. Surprisingly, the answers are closely related because both work as a result of semiconductor physics.
In discussing these topics, we are fundamentally talking about how light interacts with matter and we already know the answer. Light interacts with matter in complex, varied, and often beautiful ways. Consider a rainbow, which is the interaction of light with spherical water drops inside clouds. That is a process called refraction and spectral dispersion. Consider the mobius strip photographs in my “Manmade gallery.” Those spectacular colors are created by a process called optical birefringence.
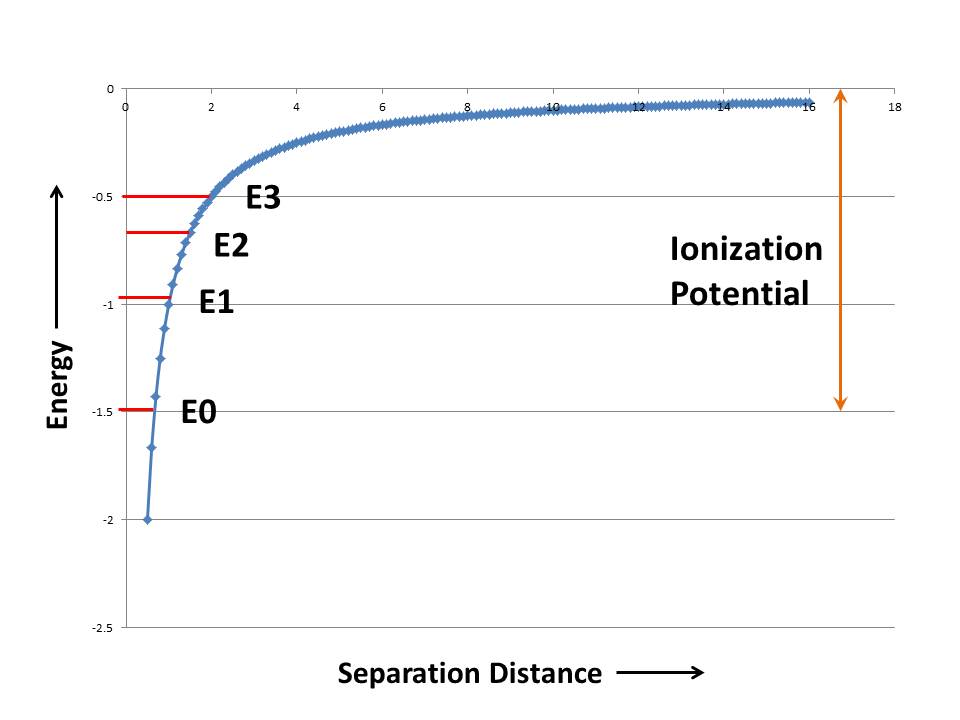
Figure 1 – The potential well or Curve of Binding Energy (c) DEWolf 2013
But let’s keep things reasonably simple here. We need to understand our subject qualitatively, not quantitatively, which means, yay, no equations!
OK, so we know that atoms are made up of protons, which are positive charges, and electrons, which are negative charges. Opposites attract. So given half a chance an electron will rush towards a proton. That’s how lightening works! Ka-boom!
Now let’s hold an electron some distance away from the proton. You will feel the force of attraction in your fingers. This is referred to as potential energy, because if you let go the electron will move very quickly towards the proton, that is there is the potential for motion, aka kinetic or motion energy. By convention, because of the attractive nature of the force, the energy is said to be negative. We can plot the energy as a function of the distance between the electron and proton. In general the electron will seek the lowest energy. That is, it will try to go to the bottom of the well. The bottom of the well here is at minus infinity, which is a very cozy place for our electron to hide out.
The well of Figure 1 is what the electron sees. An electron can only be at the bottom of the well if it has no kinetic energy. If it has kinetic energy it will be a bit higher in the well. Next, it turns out that the electron cannot have any energy value that it wants. Only very discrete and well defined levels exist. This statement about discrete states is the foundation of the science of quantum mechanics. While it may seem bizarre, it is never-the-less borne out by countless supporting experiments. It is on a very sound theoretical and experimental proof-of-the-pudding foundation.
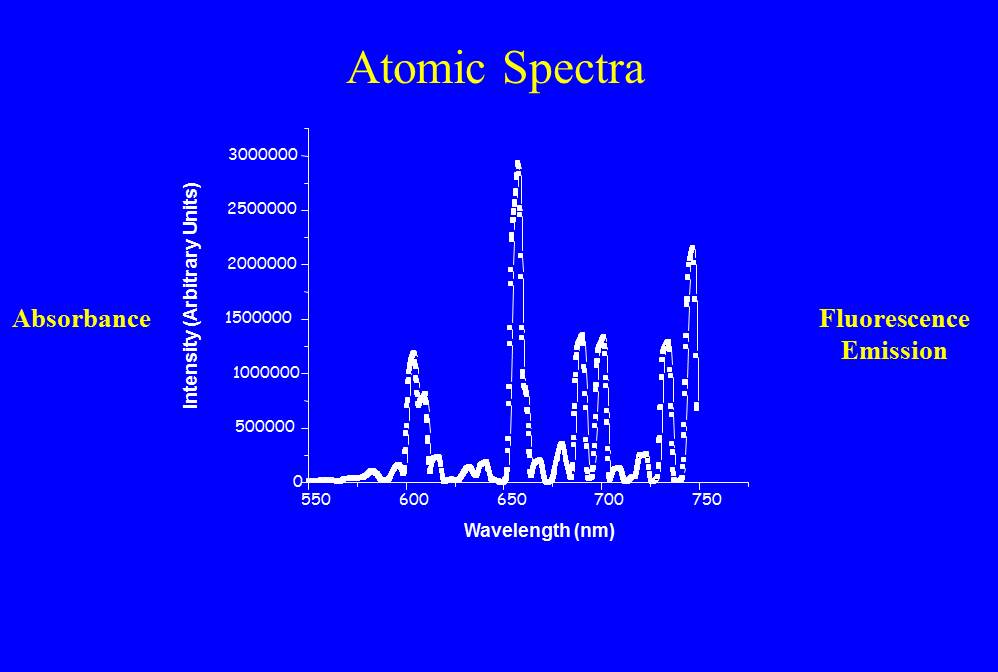
Figure 2 – Atomic spectrum of the plasma glow of a HeNe laser (c) 2013 DEWolf
In Figure 1 I’ve shown some energy levels where the electron can reside. The electron can move between these states, but only if you give it more energy, because energy has to be conserved in physics. Where does that energy come from? One possibility is light. If the photon of light has exactly the right amount energy, is exactly the right color, it can be absorbed and elevate the electron to the next allowed energy level. You can also accomplish this by heating the system up, or bombarding it with a spray of electrons. Always a precise amount of energy is absorbed, called one quanta. Often the electron falls back to the previous state. To do so it has to emit light of the precise color again. Consider Figure 2, which shows the “plasma glow” of a Helium Neon laser. The plasma glow emission is caused by transitions between lots of states and is created by showering the gas with a beam of electrons. The emitted light is a set of very sharp discrete wavelengths.
The one last critical point is that if you give the electron more than a certain amount of energy, referred to as the ionization potential, it can escape the proton all together. It becomes a free electron! I’ve shown the ionization potential (change in energy required to take the electron from it’s lowest energy level to the top of the binding curve) in orange.
In atoms that have more than one electron, the electrons sequentially fill up the energy levels. Two electron cannot exist in the same state. The term state is not quite the same as the term energy level. But that doesn’t really matter for our discussion. What is important is that the electron with the highest energy, the one that is nearest the top is the most active electron. It is referred to as “the valence” electron and is most likely to become ionized and escape from the atom or, in fact, to interact with neighboring atoms.
The Bottom Line
The important points for our discussion of light detection in photography are:
- electrons in atoms occupy specific energy levels
- the most active electrons are those with the highest energy, referred to as valence electrons
- light can cause electrons to move between energy levels
- given sufficient energy an electron can be ionized and escape from the atom